Refine Research
Explore all products that ALEA CLINICAL has to offer
Screening
Configure ALEA’s Screening and Enrollment Application to suit your trial. Generate screening numbers or have site staff enter their own. The ALEA Screening and Enrollment Application is complete and ready to use. And, you can instantly add extra fields and edit checks based on your trials’s requirement.
Randomization
The randomization service includes flexible form definition, unlimited stratification factors, unlimited number of treatments, blocked randomization, minimization, unequal allocation schemes, multi step randomisations, a simulation service which produces a comprehensive validation report, and more. It allows for re-use of components at the level of questions, forms and complete studies.
Supply Management
The Drugs Supply Management supports both simple and complex drug distributions schemes. It includes per site resupply trigger levels and sizes, per site initial shipment sizes, upload of concealment lists, generating concealments lists, predictive drug supply trough pDSM algorythms with deltaS deltaT site accrual prediction.
Electronic Data Capture
eCRF
The eCRF turnkey solution is based on ALEA™ which provides a comprehensive, rich electronic case report forms system supporting the use of electronic case report forms by the study sites as well as study coordination centers for healthcare trials. It can be used with a standard browser (IE7 up, FireFox, Chrome running on any computer connected to the internet).
ePRO
Patient self-reported data are increasingly playing a key part in efficacy and quality of life assessment, patient recruitment, symptom and safety information and medical compliance monitoring. Unlike laboratory tests and other physical assessments that require interpretation from a secondary source such as a clinician, PROs are measures that come directly from patients. Evidence shows that higher compliance can be achieved if data is collected by electronic means rather than paper. Higher compliance with ePRO is directly related to special features that electronic technologies provide, such as reminders for patients to complete diary entries and mechanisms to facilitate data completion.
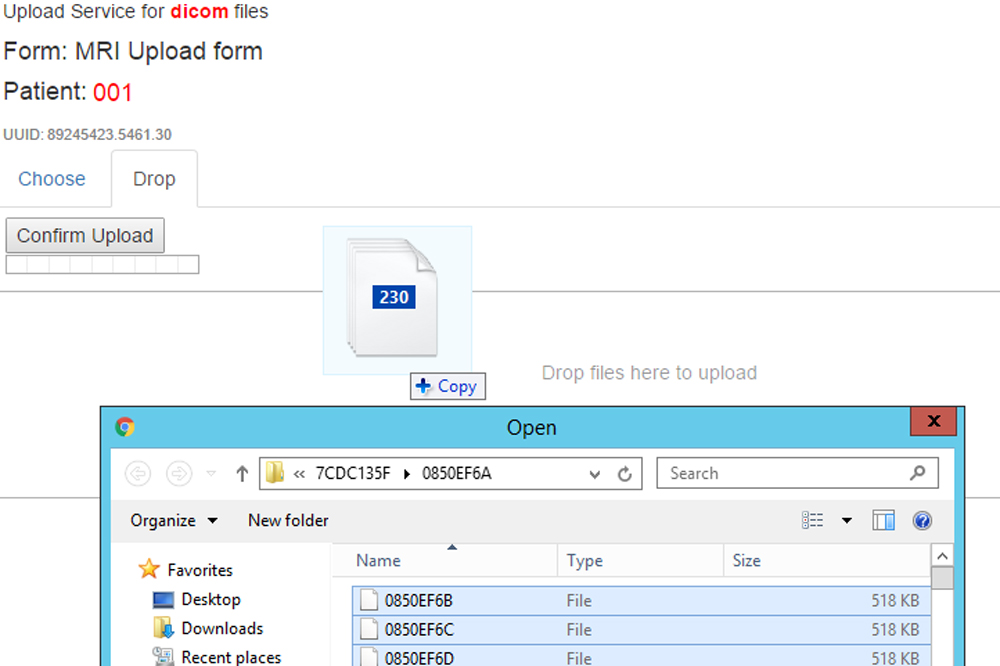
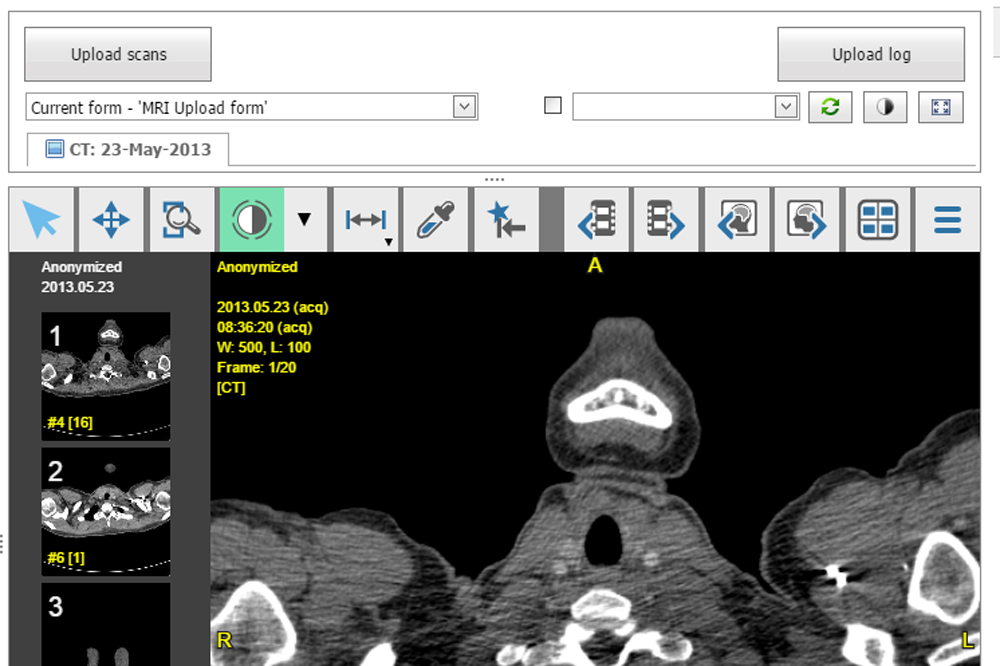
Scan Integration [new]
ALEA eCRF includes an integrated solution for incorporating imaging objects such as CT, PACS, and MRI scans into the clinical trial patient dossiers. This DICOM image viewer can be used in many different scenarios, for instance when a panel of surgeons evaluates whether a patient suffering a liver tumor os operable or not. ALEA provides an advanced notification system where for instance research nurses are notified to upload new images, or surgeons are reminded that they are requested to perform an evaluation including a link to the appropriate form to be completed.
Digital Microscophy [new]
The next stage of integration is digital microscopy imaging used in pathology. Digital microscopy produces large binary objects – varying from 100MB to over 10GB in size – which are transmitted from the clinical site participating in the clinical research to a central repository.
Digital Microscophy
The next stage of integration is digital microscopy imaging used in pathology. Digital microscopy produces large binary objects – varying from 100MB to over 10GB in size – which are transmitted from the clinical site participating in the clinical research to a central repository.
ALEA randomisation methods
ALEA randomsiation supports the following methods of randomisation for healthcare trials. It supports trials with any number of treatment groups, and randomisation ratios other than 1:1.
Minimisation
The randomised groups are balanced on the basics of the characteristics of each participant. Each new participant is allocated to the group that has the fewest number of people like him or her at that time. The default setting is that the allocation uses simple randomisation only if there is no difference between the groups when the new participant is entered into the trial.
Minimisation may be configured in accordance with the options set out in Pocock and Simon’s paper. These settings determine how much the system biases the random allocation in favor of the underrepresented treatment group passes some threshold. This method can be used to help ensure balanced the patient characteristics within each group.
Block randomisation
Participants are allocated to the treatment group that is next in a block of allocations. Within each block, each treatment group occurs the same number of times. The user selects the block size for their trial as a whole or for specific parts of their trial.
Random block randomisation
To make it less likely that the next allocation can be predicted, this method varies the size of each block. ALEA Clinical chooses the size of each block by multiplying the number of treatments groups by a whole number between 1 and a minimum block size, set by the user.
Simple randomisation
Each participant has an equal change of being allocated to any of the treatment groups, regardless of any imbalances that have already build up within the trial.
* Blinding
If participants in the trial are not to know which treatment they will receive, ALEA Randomisation can convert the treatment allocated into a box number or code, and returns this rather than the treatment. ALEA Randomisation helps the user to prepare these numbers or codes, so that the trial material can be prepared in advance.